DREAMM 6
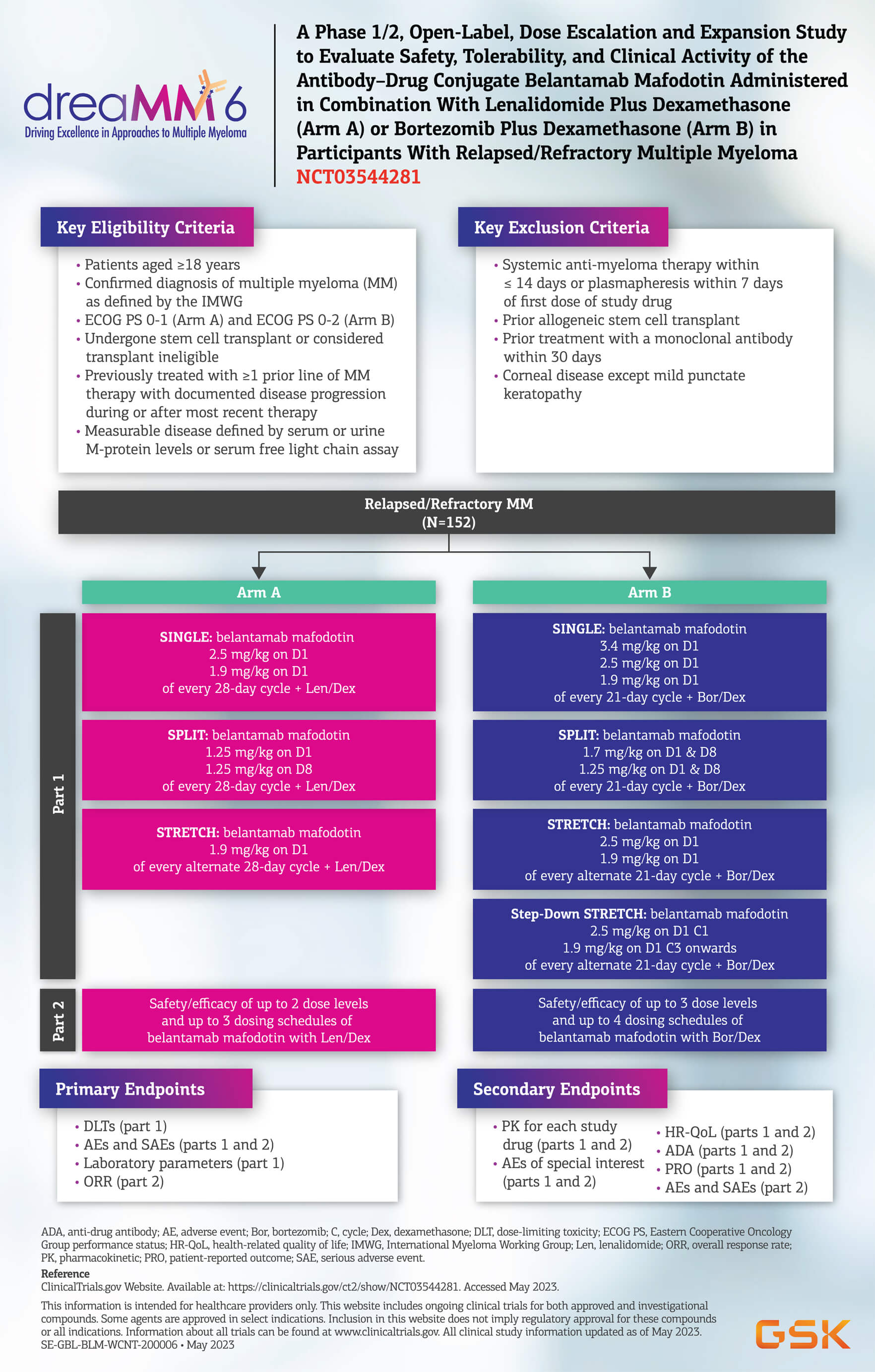
A Phase 1/2, Open-label, Dose Escalation and Expansion Study to Evaluate Safety, Tolerability, and Clinical Activity of the Antibody-Drug Conjugate Belantamab Mafodotin Administered in Combination With Lenalidomide Plus Dexamethasone (Arm A), or Bortezomib Plus Dexamethasone (Arm B) in Participants With Relapsed/Refractory Multiple Myeloma4,5
DREAMM 6
This study will evaluate the safety and tolerability profile of belantamab mafodotin when administered in combination with approved regimens of either Lenalidomide Plus Dexamethasone [Len/Dex (Arm A)] or Bortezomib Plus Dexamethasone [Bor/Dex (Arm B)] in participants with RRMM, i.e., those who have relapsed or who are refractory to at least 1 line of approved therapy. Part 1 of the study is a dose escalation phase to evaluate the safety and tolerability of up to 3 dose levels and up to 2 dosing schedules of belantamab mafodotin in combination with the two standard of care (SoC) regimens. Part 2 will further evaluate the safety and preliminary clinical activity of belantamab mafodotin at selected dose levels and dosing schedules in combination with Len/Dex or Bor/Dex.
Up to a total of 152 evaluable participants will be enrolled in the study with up to 27 in Part 1 and up to 125 in Part 2. Participants receiving treatment Arm A, may continue combination treatment until the occurrence of progressive disease (PD), intolerable adverse events (AEs ), consent withdrawal, or death. The participants receiving treatment Arm B, may continue combination treatment for a total of up to 8 cycles. After 8 cycles of combination therapy, the participants will continue treatment with belantamab mafodotin, as a monotherapy until the occurrence of PD, intolerable AEs, consent withdrawal, or death.
Belantamab Mafodotin is there for you
Help us drive excellence in approaches to multiple myeloma with the support, tools, and educational resources for anyone considering belantamab mafodotin.
DREAMM 6 currently active, not recruiting with an estimated study completion date of Febuary 28, 2024
Other Trials You May Be Interested In
A Phase 1/2, Randomized, Open-label Platform Study Utilizing a Master Protocol to Study Belantamab Mafodotin as Monotherapy and in Combination With Anticancer Treatments in Participants With Relapsed/Refractory Multiple Myeloma3
A Multicenter, Open-Label, Randomized Phase 3 Study to Evaluate the Efficacy and Safety of the Combination of Belantamab Mafodotin, Bortezomib, and Dexamethasone (B-Vd) Compared With the Combination of Daratumumab, Bortezomib and Dexamethasone (D-Vd) in Participants With Relapsed/Refractory Multiple Myeloma6
A Phase 3, Multicenter, Open-Label, Randomized Study to Evaluate the Efficacy and Safety of Belantamab Mafodotin in Combination With Pomalidomide and Dexamethasone (B-Pd) Versus Pomalidomide Plus Bortezomib and Dexamethasone (PVd) in Participants With Relapsed/Refractory Multiple Myeloma7
A Phase 1, Randomized, Dose and Schedule Evaluation Study to Investigate the Safety, Pharmacokinetics, Pharmacodynamics and Clinical Activity of Belantamab Mafodotin Administered in Combination With Standard of Care in Participants With Newly Diagnosed Multiple Myeloma8
A Phase 1 Study to Evaluate the Pharmacokinetics and Safety of Belantamab Mafodotin Monotherapy in Participants With Relapsed or Refractory Multiple Myeloma Who Have Normal and Varying Degrees of Impaired Renal Function9
A Phase 1 Study to Evaluate the Pharmacokinetics and Safety of Belantamab Mafodotin Monotherapy in Participants With Relapsed or Refractory Multiple Myeloma Who Have Normal and Varying Degrees of Impaired Hepatic Function10
A Phase 2, Randomized, Parallel, Open-label Study to Investigate the Safety, Efficacy, and Pharmacokinetics of Various Dosing Regimens of Single-agent Belantamab Mafodotin in Participants With Relapsed or Refractory Multiple Myeloma11
